Description
SUMO Protease recognizes SUMO tertiary structure – not a specific amino acid sequence- and cleaves after the C-terminal glycine at the end of the SUMO sequence. If the target protein is fused downstream of the SUMO C-terminus, SUMO Protease cleavage renders the native protein sequence without any additional amino acids.
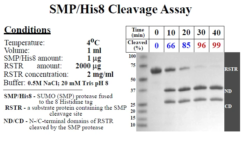
Cleavage Efficiency: 1 ug protease will digest 2 mg protein with 99% efficiency at 4°C in 40 minutes.
The his-tagged SUMO protease can be used to release target proteins from TriAltus’ Im7 resin. The protease cleaves and releases the target protein from the Im7-bound CL7 tag. The final protease concentration in the eluted sample comprises ~1.5-3%. If needed, SUMO Protease can be removed from the sample with a His-trap column or via size exclusion chromatography.