CL7/lm7 Protein Purification System

Clean, tag-free protein in a single chromatography step with the new CL7 ultra-high affinity tag and its Im7 ligand
- Up to 99% purity from one round of purification
- As much as 80 x higher yield than his-tag
- Tag-free protein by on-column protease elution
- Ideal for high-throughput protein purification and for difficult-to-isolate proteins, e.g. membrane proteins and multi-subunit complexes
- Meets high-yield, high-purity, and high-activity (HHH) requirement of high-resolution structural analysis as well as industrial protein production
The CL7/Im7 system is based on an ultra-high-affinity interaction between a variant of the Colicin E7 DNAse (CL7) and its inhibitor, Immunity protein 7 (Im7) (Vassylyeva et al.: Efficient, ultra-high-affinity chromatography in a 1-step-purification of complex proteins,
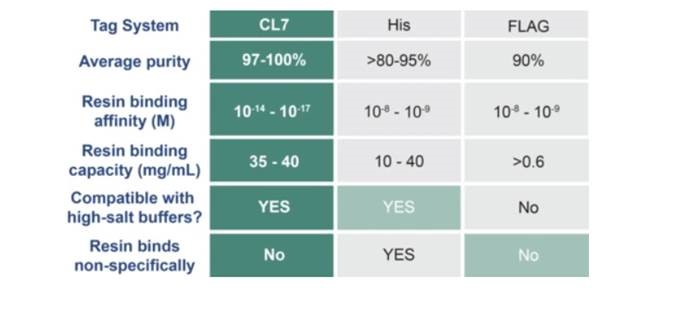
The ultra-high-affinity results in superior properties versus other tag-based systems.
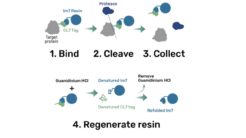
Workflow:
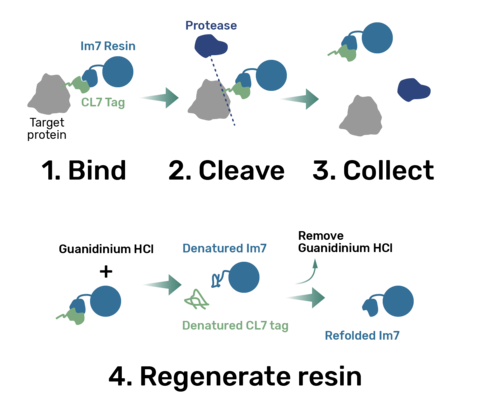
1. Bind
The protein is expressed in a CL7-tagged vector of your choice (see below for a variety of mammalian and E. coli vectors with C- or N-terminal CL7 tag and additional tags, e.g. trx1 for improved protein folding, or signal peptides for membrane protein expression). Cellular lysate is applied to the lm7 resin which is available activated in a slurry format or pre-loaded in gravity resp. FPLC columns (see below) and consists of 6B or 4B agarose beads which are covalently linked to the Im7 ligand.
4B agarose beads are recommended for large proteins (e.g. TriaAltus´40 kD test protein shows a binding capacity of ~60 mg/mL with 4B resin vs ~40 mg/mL 6B resin and Cas9 binds at ~12 mg/mL 4B resin vs ~8 mg/mL 6B resin), but it should be noted that the flow rate is slower and the column backpressure is higher, so for standard size proteins 6B resin is preferable.
2 & 3. Cleave and Collect
The CL7-tagged expression vectors include cleavage sites to remove the CL7 tag (and other tags if applicable) by either Prescission Protease or SUMO Protease. After on-column protease cleavage the tag free clean protein is eluted from the column.
4. Regenerate
The lm7 resin can be regenerated with Guanidinium HCl and re-used up to 100 times.
Purification Data:
The TriAltus CL7/lm7 system has been used successfully for many different proteins including difficult-to-purify ones such as membrane proteins, DNA/RNA-binding multi-subunit proteins and proteins toxic to E. coli.
Membrane Proteins:
Coomassie stained gels comparing His-trap and CL7/Im7 purification of YidC and CNX:
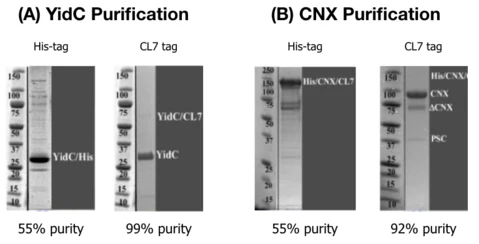
Multi-Subunit, DNA-Binding RNAPs:
Coomassie stained gels comparing His-trap and CL7/Im7 purification of ttRNAP and showing one-step Im7 purification of mtRNAP:
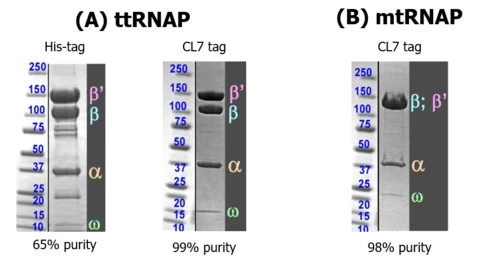
Toxic Proteins:
Proteins which are toxic to E. coli cannot be over-expressed in the host by nature. The sensitivity of the CL7/lm7 system enables the purification of such difficult proteins at excellent purity, as shown here:
The highly toxic bacterial MukBEF condensin (~250 kDa, 3 subunits) was successfully purified by one step CL7/Im7 purification.
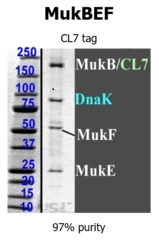
Biosimilar Proteins:
TriAltus´ CL7/lm7 system has been extensively used to purify human therapeutically relevant proteins in large quantities and high purities from E. coli. See Coomassie stained gels of human NEF, Growth Hormone, IFNa, GCSF, and Fc, all highly purified with the TriAltus system:
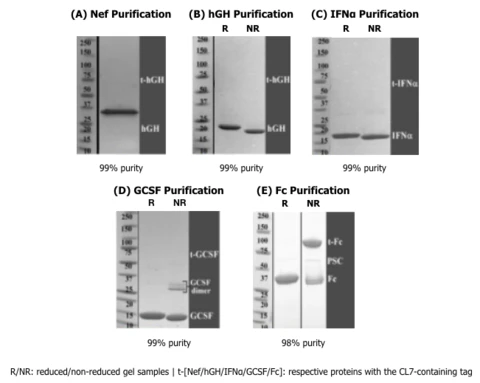
CAS9 Proteins:
Purity of Cas9 highly impacts CRISPR-based genome editing using the RNP approach. The CL7/lm7 system enables the one step purification of highly pure and active Cas9 in contrast to the traditionally used his tag purification:
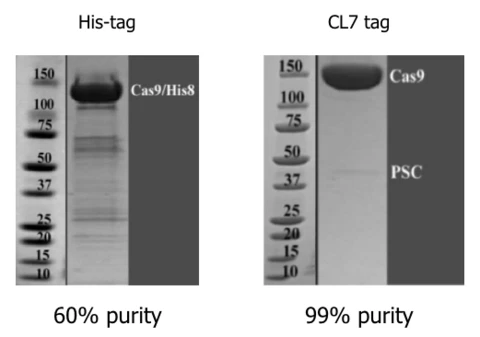
Researchers at Hubei University (Wuhan, China) used Trialtus´ CL7/Im7 system to purify even Cas9 ribonucleoproteins (RNPs) in one step (Qiao et al.: Co-expression of Cas9 and single-guided RNAs in Escherichia coli streamlines production of Cas9 ribonucleoproteins. Commun Biol 2, 161 (2019).
Showing 1–20 of 34 results